
Electrochemistry Class 12 Important Questions with Answers
View Electrochemistry Class 12 PPTs online, safely and virus-free! Many are downloadable. Learn new and interesting things. Get ideas for your own presentations. Share yours for free!

PPT AP Chemistry Unit 12 Electrochemistry PowerPoint Presentation
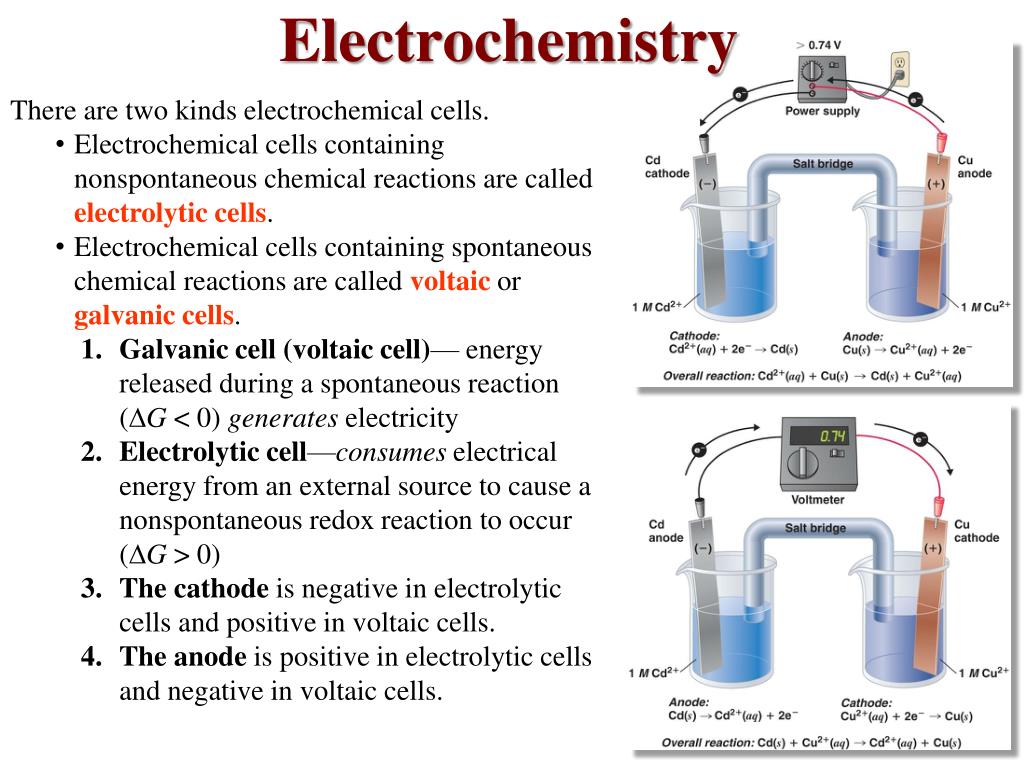
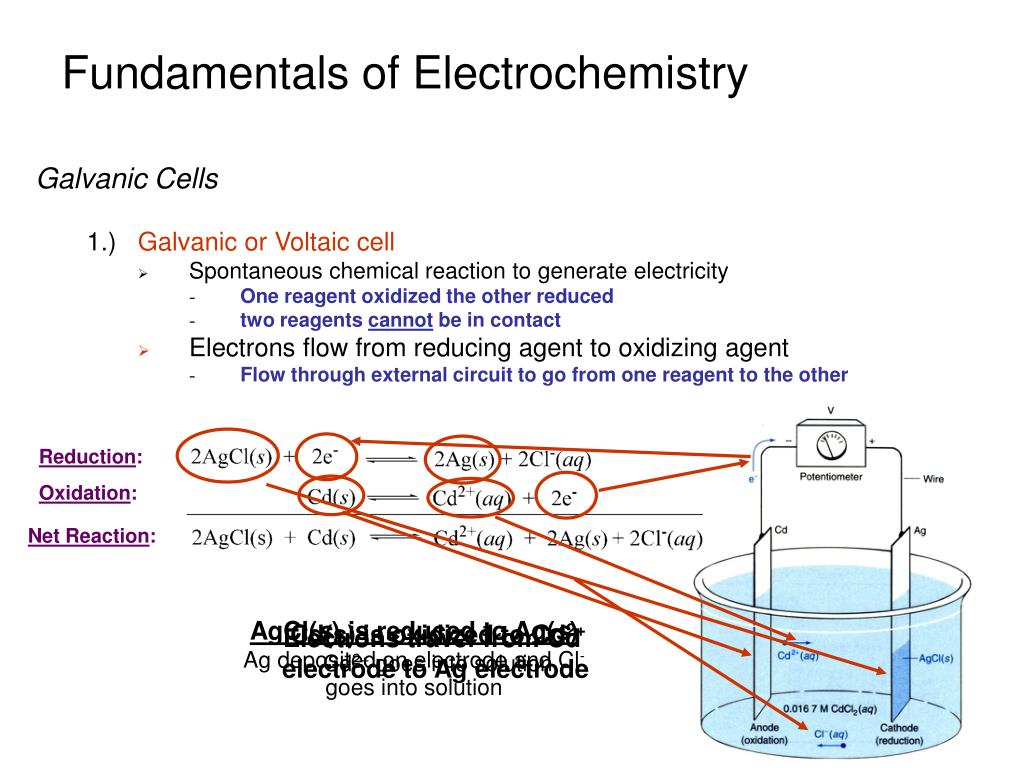
These are two fundamental types of electrochemical cells. Galvanic cell - In a galvanic cell, spontaneous redox reaction, the chemical energy is converted into electrical energy. It is also called a voltaic cell or Daniel cell. Electrolytic cell - In an electrolytic cell, the nonspontaneous redox reaction is carried out by electrical energy.

Electrochemistry Class 12 Mind Map
NCERT Solutions for Class 12 Chemistry Chapter 3 - Free PDF Download. NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry play a pivotal role in the CBSE Class 12 Chemistry board examination.NCERT Solutions for Class 12 Chemistry are comprehensive materials that have answers to the exercise present in the NCERT Textbook. These solutions are developed by subject experts at BYJU.
Ppt Basic Electrochemistry Powerpoint Presentation, Free Download E45
Electrochemistry class 12 Apr 11, 2018 • 65 likes • 58,226 views Cleophas Rwema Education Electrochemistry Electrochemistry class 12 1 of 68 Download Now Save slide Save slide Recommended d and f block elements Tinto Johns Vazhupadickal 66.2K views • 75 slides Classification of elements and periodicity in properties class 11th chapter 3. ritik

electrochemical cell electrochemistry class 12 chemistry subject notes
These metallic electrodes are immersed in an electrolytic solution for power generation. By thorough reading of chapter 3 Chemistry class 12 notes, students will know that the ionic conductor is a vital part of cells. Download CBSE Class 12 Chemistry Notes 2023-24 PDF. Also, check CBSE Class 12 Chemistry revision notes for other chapters:

Chapter 3 Electrochemistry Class 12 Handwritten Notes PDF download
Class XII Electrochemistry - Download as a PDF or view online for free. Submit Search. Upload. Class XII Electrochemistry . Report. Share. Arunesh Gupta Govt. service at KVS, New Delhi.. Chapter 8 redox reactions ppt for class 11 CBSE by .
CLASSNOTES Electrochemistry Notes For Class 12 Free Download
Electrochemistry Nov 28, 2014 • 113 likes • 82,809 views Pratik Sudra Production-Executive at UPL,unit1,Ankleshwar Electrochemistry 1 of 41 Download Now Save slide Save slide Recommended Transition metal geetha T 12.9K views • 58 slides Electrochemistry-Dr. Surendran Parambadath Surendran Parambadath 9.7K views • 89 slides
PPT Electrochemistry PowerPoint Presentation, free download ID1195570
12th Chemistry PowerPoint Presentation Materials (Complete PPT Materials) Mr. B. Uthrakumar. Unit 1 PPT ( Metallurgy) - English Medium - Preview & Download (MAT.NO. 219313) Unit 2 PPT (p Block Elements I) - English Medium - Preview & Download (MAT.NO. 219394) Unit 3 PPT (p Block Elements II) - English Medium - Preview & Download.
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free
Get the complete notes of Chapter Electrochemistry of Class 12 specially prepared by the experienced faculties of physics wallah, these notes will help students to understand the concepts and use them at the time of revision. Slideshow 11592774 by maitri3

Electrochemistry Class 12 One Shot CBSE NEET JEE Chapter 3 YouTube
PPT: Electrochemistry - Chemistry Class 12 - NEET Download, print and study this document offline Download as PDF Up next NEET Previous Year Questions (2014-23): Electrochemistry NCERT Solutions: Electrochemistry Flashcards: Electrochemistry Top Courses for NEET Biology Class 11 NEET Mock Test Series Topic-wise MCQ Tests for NEET Physics Class 11

Electrochemistry class 12 notes [PPT Powerpoint]
Electrochemistry is that branch of chemistry which deals with the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations. Importance of Electrochemistry Production of metals like Na, Mg. Ca and Al. Electroplating.

Electrochemistry Notes for Class 12, IIT JEE & NEET
Electrochemistry Electrolysis ' Electrolysis: is the process in which electrical energy is used to drive a nonspontaneous chemical reaction. An electrolytic cell is an apparatus for carrying out electrolysis. ' Processes in an electrolytic cell are the reverse of those in a galvanic cell. Slide 2

Electrochemistry class 12
In Class XI, Unit 8, we had studied the construction and functioning of Daniell cell (Fig. 3.1). This cell converts the chemical energy liberated during the redox reaction Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s) (3.1) to electrical energy and has an electrical potential equal to 1.1 V when concentration of Zn2+ and Cu2+ ions is unity (1 mol dm-3)*.

ELECTROCHEMISTRY PART3 CLASS12 YouTube
Notes of 11 - 12, Chemistry 3 Electrochemistry presentation.pdf - Study Material. Page 29 : Applications of Electrochemical series, , 1.Comparing the relative oxidising and, reducing powers, Substances with higher reduction potentials are strong, oxidising agents, Fluorine has the highest electrode potential thus, Fluorine gas is the strongest oxidising agent, Substances with lower reduction.

Chapter 3 Electrochemistry Class 12 Handwritten Notes PDF download
This portion of power point presentation on electrochemistry includes, electrolytic conduction, conductivity, cell constant, molar conductivity, variation with concentration & temperature, Debye Huckel Onsager equation, Limiting molar conductivity, Kohlrausch law of independent migration of ions, its application & numerical problems.

PPT Chapter 7 Electrochemistry PowerPoint Presentation, free download
anode positive. A student places a copper electrode in a 1 M solution of CuSO4 and in another beaker places a silver electrode in a 1 M solution of AgNO3. A salt bridge composed of Na2SO4 connects the two beakers. The voltage measured across the electrodes is found to be + 0.42 volt. (a) Draw a diagram of this cell.